Cullgen’s Novel E3 Ligands
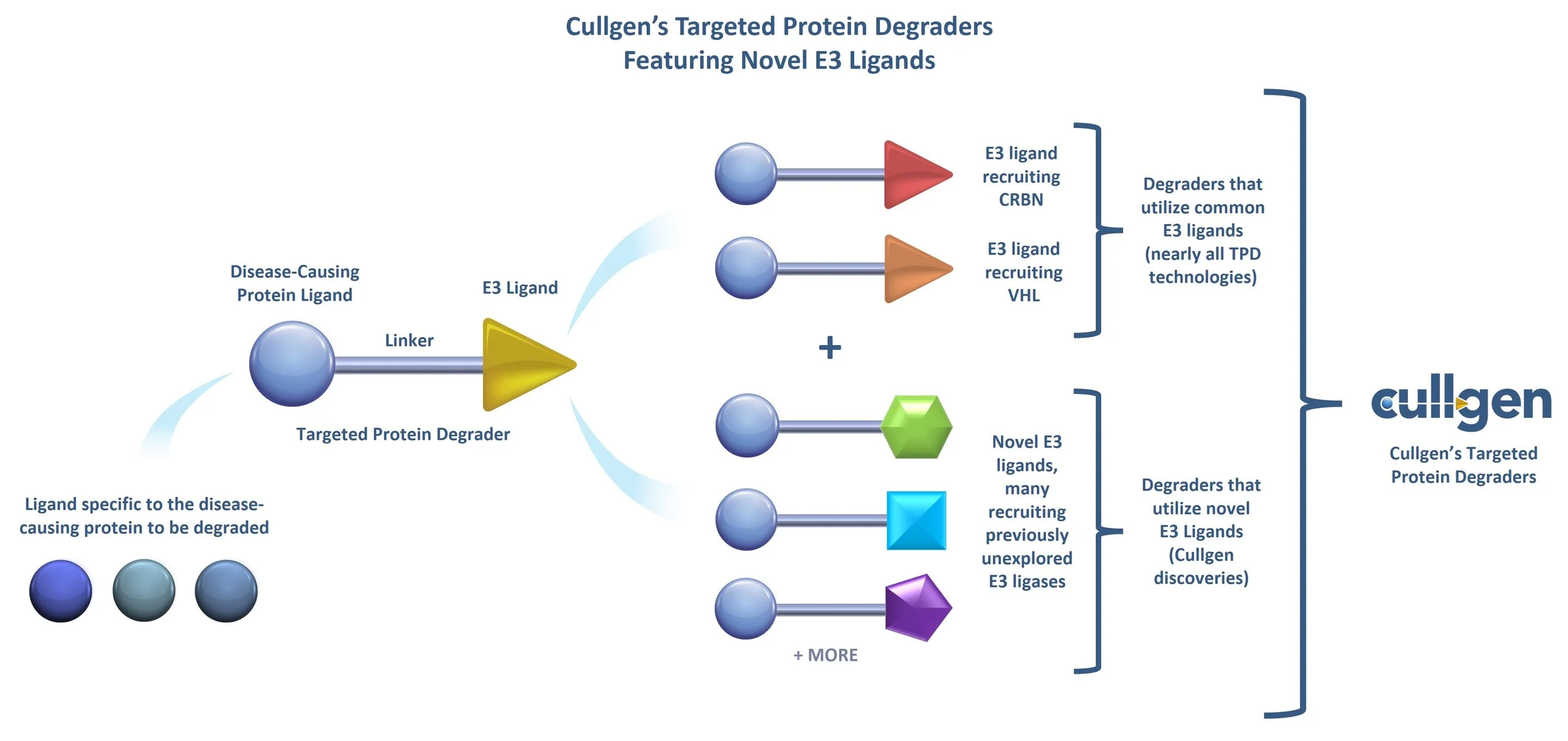
Nearly all targeted protein degradation technologies utilize E3 ligands that recruit either the CRBN or VHL E3 ligase. However, like many other E3 ligases, CRBN and VHL are not functionally essential in humans. Tumor cells have the capacity to mutate or turn off the expression of the genes encoding these ligases, rendering the corresponding degrader ineffective.
Cullgen has discovered multiple novel E3 ligands, many of which recruit previously unexplored E3 ligases, including those that are functionally essential, meaning their expression is not likely to be affected by tumor cells. This provides a significant competitive advantage for degraders used in cancer applications.
In addition to utilizing functionally essential E3 ligases, Cullgen has also discovered E3 ligands that target previously unexplored E3 ligases that exhibit tissue-selective or tumor-enriched properties. The ability to utilize tissue-selective E3 ligases means that Cullgen can develop degraders that can actively target or avoid specific issues, and by targeting tumor-enriched E3 ligases, Cullgen can design and develop degraders that are safer and more efficacious.
Please watch the video below to learn more about Cullgen’s novel E3 ligands.
Video created by life-science-animation.com
-
The ubiquitin-proteasome system in humans includes more than 600 different E3 ligases. Nearly all targeted protein degraders in development today utilize E3 ligands that recruit either the Cereblon or the VHL E3 ligase for degrading disease-causing proteins. However, they, and many other E3 ligases, are not functionally essential in humans. When used in cancer applications, tumor cells may mutate or turn off the expression of the genes encoding them, rendering the corresponding degrader ineffective.
Cullgen has gained proprietary knowledge to optimize both ends of the bifunctional degrader molecule. One end binds to the disease-causing protein while the other recruits a specific E3 ligase selected from a range of E3 ligases, including the ones that have been previously unexplored, some of which are functionally essential, providing a competitive advantage when used in cancer applications.
Cullgen also has the capability to discover novel E3 ligands that recruit previously unexplored tissue-selective or tumor-enriched E3 ligases. By discovering E3 ligands that recruit tissue-selective E3 ligases, Cullgen can develop degraders that target or avoid specific tissues. And by developing E3 ligands that recruit tumor-enriched E3 ligases, Cullgen can develop degraders that can be safer and more efficacious.
Learn more at Cullgen.com
Novel E3 Ligands Represent the Future of Targeted Protein Degraders
At Cullgen, we believe the future of targeted protein degraders lies in the discovery and utilization of novel E3 ligands. We have identified and found numerous E3 ligands to several previously unexplored E3 ligases that are either functionally essential, tissue specific, or tissue restrictive. Access to such E3 ligases provides Cullgen with additional tools to selectively degrade disease-causing proteins.
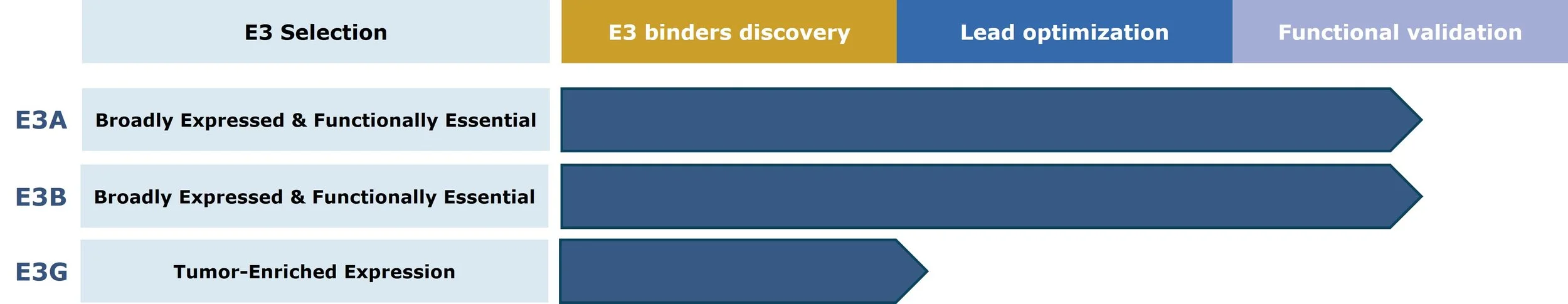
Our pipeline of novel E3 ligands is shown below. For more detail regarding our work on novel E3 ligands, click here.
Cullgen’s Novel E3 Ligand Discovery Pipeline